Chemistry
When you land on the right solution for more effective and engaging chemistry labs, your reaction should be joy! Our Quality Matters-certified chemistry discipline includes more than 65 hands-on and virtual labs, with lessons on observing chemical reactions, building molecular models, performing titrations, and identifying unknown chemicals, just to name a few. All of our labs are based on extensive academic research, developed by scientists, and customizable to suit your needs so you can be confident you’re delivering rigorous, high-quality labs online or on-campus.
Schedule a 15 minute intro call with our education specialist to learn more!
Trusted by over 1,000 institutions of all sizes




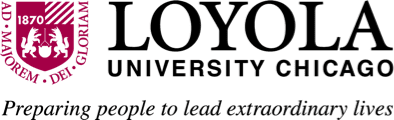

Enriching on campus, hybrid & online courses
Whether your course is fully online, on campus or hybrid, our chemistry labs can help you elevate the learning experience for all students and reinforce critical concepts by:
- Better preparing students with digital pre-labs before in-person labs
- Using at-home hands-on labs to create an experience comparable to on campus, helping students build practical skills applicable for advanced studies
- Supplementing hands-on labs with digital lessons in topics like chromatography, acid base titration, and stoichiometry
- Balancing time spent on campus for hybrid students with convenient, accessible & efficient ways to engage in quality learning experiences
Delivering interactive chemistry lab experiences has never been easier.
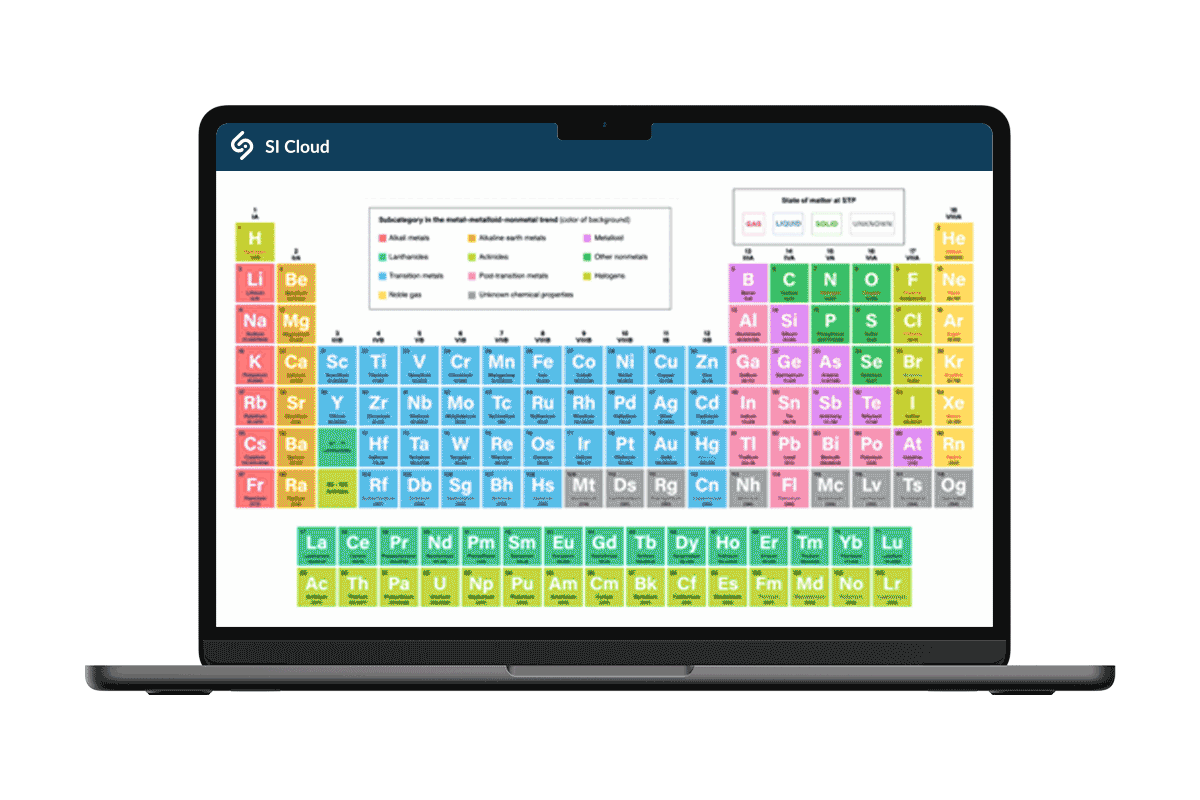
Hands-on labs for effective, high quality online courses
When you want to more deeply engage students and create a learning experience comparable to the on-campus experience, be sure to incorporate hands-on labs.
- Foster a more authentic and engaging lab experience by mirroring the quality of a campus lab when you have students physically conduct experiments
- Instill and reinforce the knowledge and critical thinking skills students need to pursue additional coursework in chemistry
- Increase the likelihood students can use your course for transfer credit if needed
- Implement quickly and easily into your current courses
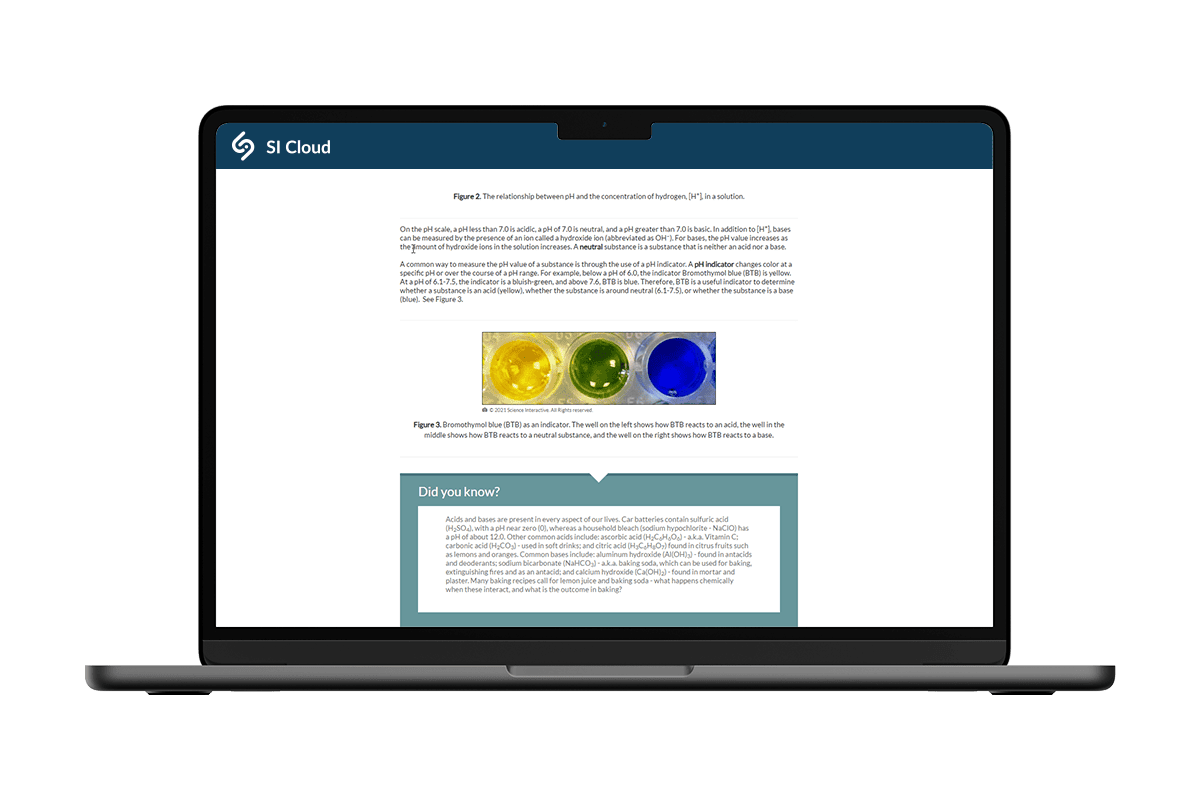
Digital chemistry labs for greater flexibility & affordability
When you want to balance costs, engage students in new ways, and reinforce key concepts in a low-stakes way, turn to our virtual chemistry labs.
- Increase access & affordability without the need for expensive or large-scale lab equipment
- Use multiple, different labs to expose students to complex and hard-to-observe phenomena
- Minimize any potential safety concerns & ensure practicality
- Implement quickly and easily into your current courses
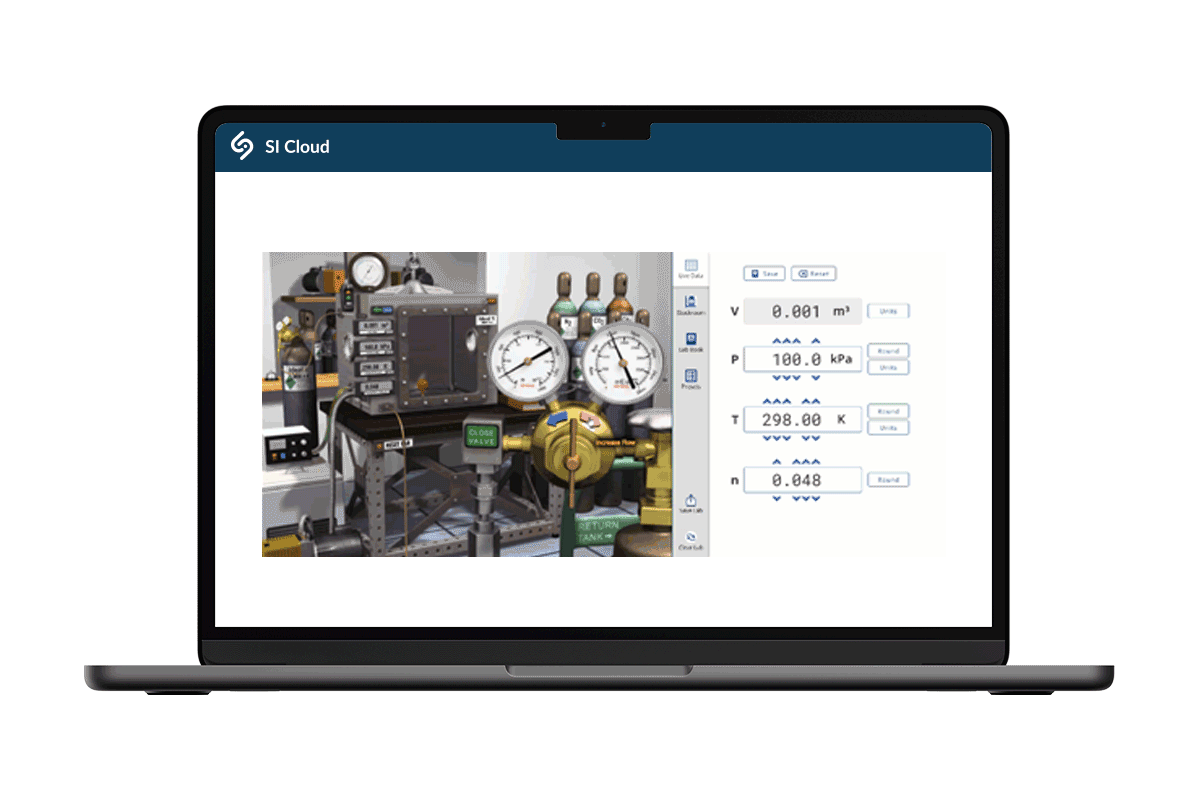
Rigor is foundational!
Whether digital or hands-on, our chemistry labs are produced with quality and rigor in mind. And unlike other providers, our chem labs are:
- Built on extensive academic research, developed by Ph.D. scientists, and peer-reviewed by active chemistry professors
- Tested by in-house instructional designers for quality, accuracy, safety, and ADA compliance
- Focused on today’s innovative technology using lab-grade materials/equipment in order to encourage student exploration and career readiness
- Created for both majors and non-majors courses
Why Science Interactive?
Seamless integration
Easier grading & course administration for instructors and quicker access for students with our seamless LMS integration.
Integrated OpenStax textbooks
Give students easy and affordable access to text with OpenStax’s high-quality, peer-reviewed textbooks integrated within our lab management platform.
Ease of use
Automatic grading
Assessment analytics
Identify students who may be falling behind in certain areas, giving you the opportunity to provide extra guidance to get back on track.
