


Hands-on General, Organic & Biochemistry Labs
With our broad range of innovative hands-on labs, your students can investigate a wide range of topics, including organic compounds, melting points, chromatography of food dyes, calories, and more. With Science Interactive, you can:
- Create an active learning environment online that promotes deeper student engagement, critical thinking, and problem solving.
- Give students the chance to use lab-grade materials and equipment that match the sophistication of a formal lab facility.
- Build hands-on kits that suit your needs and budget.

Virtual General, Organic & Biochemistry Labs
Similarly, our virtual general, organic, and biochemistry labs can supplement hands-on labs that require expensive equipment and be used to cover fundamental concepts like the scientific method and equation or formula work. You might also consider using virtual GOB labs as pre-lab work for in-person labs in order to better prepare students. From a simulation illustrating equilibrium and LeChatelier’s Principle or osmotic pressure, students can run virtual GOB labs an unlimited number of times in order to:
- Manipulate different variables, re-run the experiment, and see different results, especially important for struggling students.
- Reinforce critical learning outcomes while exploring additional concepts that may not directly align with your course objectives.
- Ensure practicality when it comes to costs and time.

Effective, High-Quality General, Organic & Biochemistry Curriculum
Science Interactive develops the highest quality online curriculum for both hands-on or virtual forensic science labs. Lesson are:
- Developed by PhD scientists, with measurable learning outcomes, detailed experimental protocols, and questions that tie into the core learning objectives.
- Reviewed by instructional designers for quality, accuracy, safety, and ADA compliance — and peer-reviewed by active professors for content accuracy.
- Certified by Quality Matters to ensure the same standards of rigor and quality as on-campus GOB courses.
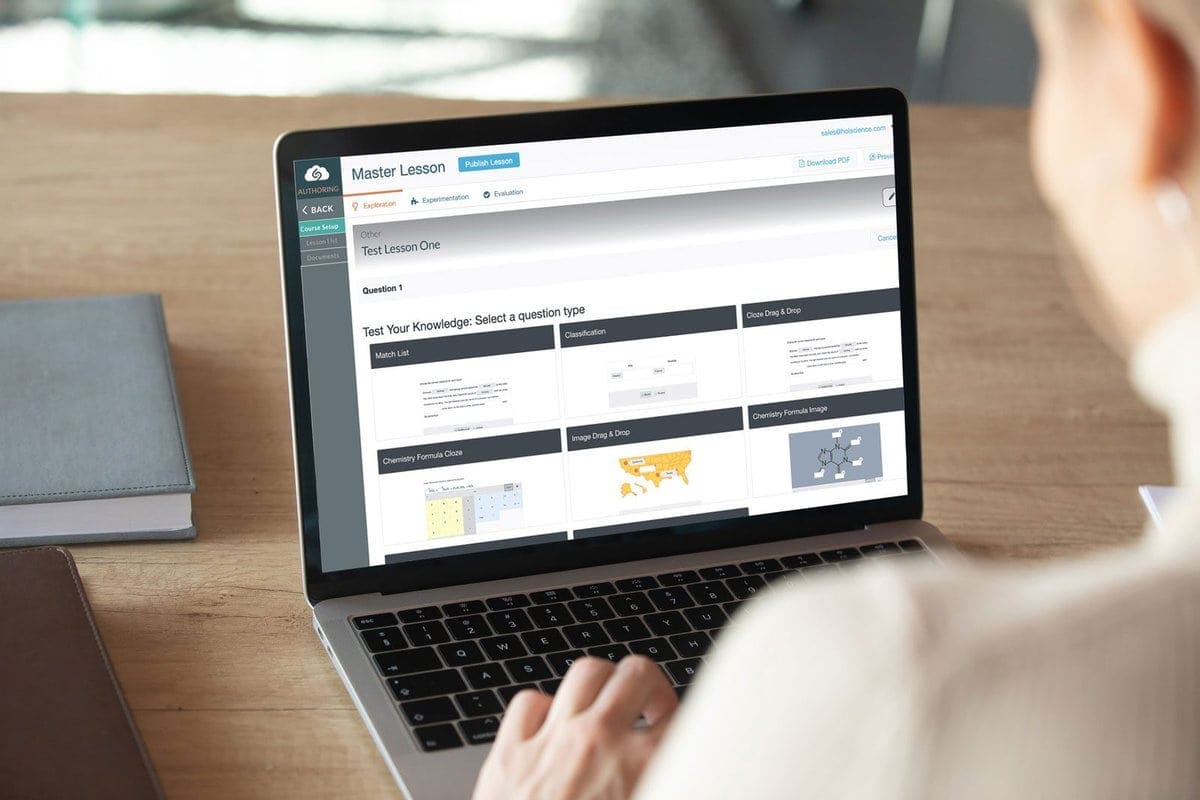
Flexible Platform to Guide Students through General, Organic & Biochemistry Labs
All of the curriculum is turnkey and can be used in your course right away. We also make it easy to customize, infusing each lab with your own unique instructional style and outcomes. Our LMS-integrated lab management platform allows you to:
- Customize any part of a lesson by adding or removing text, images, and videos to enhance lessons and guide students through each GOB lab.
- Assess student work with automatic grading for multiple choice questions.
- Provide constructive feedback so each student can continue improving for the next lab.